
Generation Of Novel, Integrated and Internationally Harmonised Approaches for Testing Metabolism Disrupting Chemicals
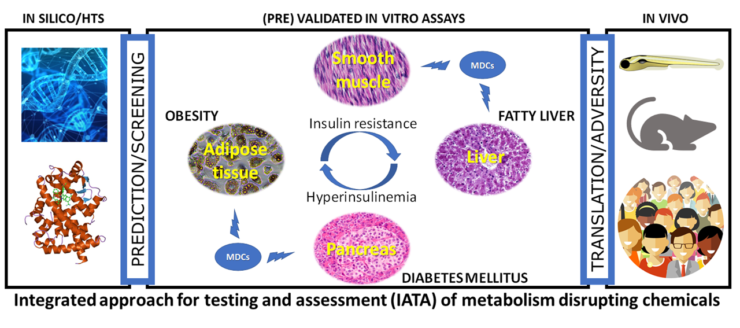
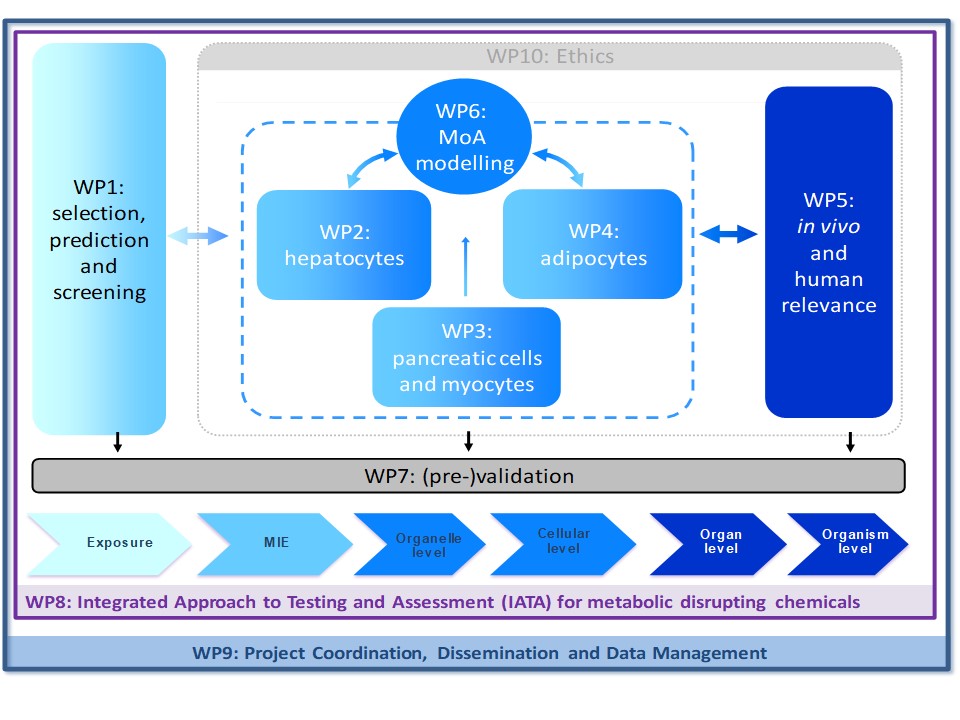
GOLIATH focusses on one of the most urgent regulatory needs in the field of endocrine disrupting chemicals, namely the lack of methods for testing EDCs that disrupt metabolism – chemicals collectively referred to as ‘metabolism disrupting chemicals’ (MDCs). MDCs are natural and anthropogenic chemicals that have the ability to promote metabolic changes that can ultimately result in obesity, diabetes and/or fatty liver in humans. GOLIATH will generate the world’s first integrated approach to testing and assessment (IATA) specifically tailored to MDCs. With a focus on the main cellular targets of metabolic disruption – hepatocytes, pancreatic endocrine cells, myocytes and adipocytes – GOLIATH will develop new methods and optimise existing methods that span the entire adverse outcome pathway (AOP) spectrum, using in silico predictive modelling and high throughput screening, (pre-)validated ready-to use in vitro assays and optimised in vivo toxicity testing guidelines. GOLIATH will provide key information on the endocrine mode of action by which MDCs disrupt metabolic pathways and induce adverse effects on human health by incorporating multi-omics technologies, and translating results from in vitro and in vivo assays to adverse metabolic health outcomes in humans at real life exposures. Given the importance of international acceptance of the developed test methods for regulatory use, GOLIATH will link with ongoing initiatives of the OECD for test method (pre-)validation, IATA and AOP development. With a consortium comprised of world-leading experts in endocrinology, molecular biology, toxicology, epidemiology, test method development, validation and chemical regulation, GOLIATH will be pivotal in the development of an internationally harmonised strategy for testing MDCs, and help to reduce the worldwide rise in metabolic disorders that have reached ‘Goliathan’ proportions.
GOLIATH Objectives
-
1. To improve understanding of the endocrine modes of action of metabolism disrupting chemicals.
We will elucidate the role of nuclear receptors (NR) and identify novel modes of action (MoA) as well as confirm suspected MoA for a panel of MDCs using in silico predictive models, in vitro screening and profiling methods. These will be optimized by the use of advanced metabolic network profiling and modelling approaches based on metabolomics and lipidomics, for the characterization of relevant diagnostic biomarkers in humans.
-
2. To develop assay candidates for metabolic disrupting chemicals based on confirmed MoA and key biological effects in target tissues.
Following a tiered approach (See figure below), GOLIATH will develop in silico quantitative structure-activity relationship (QSAR) predictive models, in vitro screening methods and dedicated in vitro assays in liver and endocrine pancreas cells as well as myocytes and models of human adipocyte differentiation. In vivo tests will be based on development of a novel alternative zebrafish model for metabolic disruption, and optimisation of existing rodent test guidelines. The selection of MDCs to be tested is based on human exposure, making the best use of current advances in human biomonitoring.
-
3. To select and develop assay candidates into (pre-)validated test methods.
The in vivo and human relevance of the developed assay candidates will be determined with epidemiological studies by read-across from in vitro endpoints to relevant markers and pathways that have been assessed in human studies. The most promising and relevant in vitro candidates will be selected and further undergo (pre-)validation according to OECD guidance and in collaboration with OECD, ensuring test method definition, transferability, inter-laboratory testing and assessment of predictivity, which are prerequisites for their regulatory use.
-
4. To develop an internationally harmonised, integrated approach to testing and assessment (IATA) of MDCs.
GOLIATH will develop the first IATA for MDCs based on the synthesis of existing information coupled with new information generated in the project, using an Adverse Outcome Pathway (AOP) conceptual framework. The IATA will be internationally harmonised through submission to the OECD case studies programme, and extensive consultation with international stakeholders. The evidence base generated in GOLIATH (human exposure, delineation of key mechanisms and MoA, key event relationships, integration of novel/existing test methods to assess MD) will provide a sound basis for hazard and risk assessment of MDCs that are currently not being adequately addressed.
More project information can be found at: https://cordis.europa.eu/project/id/825489